Oceans choke on carbon - Global extinction risks
Almost everything we consume on this planet ends up in the ocean; from the food we eat, to the manufactured products we consume, to the energy we produce.
Our use of fossil fuels, our dependence on fertilizers, our demand for more and more land, contribute one way or another to one of the greatest threats facing our ocean today, acidification.
We monitor acidification by testing the ocean’s pH scale; a measure of 0 to 14, indicating the alkaline or acidic composition of a solution. Pure water has a pH of 7, battery acid, a pH of 0.8. The ocean averages between 7.9 and 8.2pH.
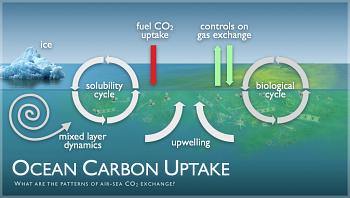
Until recently, the ocean has managed to maintain a balance between the amount of carbon it absorbs and the amount of oxygen it produces. Now our actions, especially our reliance on fossil fuels, have upset this crucial equilibrium.
We are dumping carbon dioxide into the atmosphere, at a rate of 28 million metric tons per year. This excess carbon interacts with other ions in the water to produce carbonic acid. The more acidic the water, the less ability it has to absorb CO2. Scientists fear the ocean could reach a saturation point. Increased acidity also results in fish starved for oxygen, corals and other marine species deprived of the calcium necessary to grow and spawn, and a proliferation of dead zones.
Reference: Nature of Things - CBC.ca
Comments
There are 0 comments on this post